
Treatment with VYONDYS 53 (golodirsen).
A lack of dystrophin causes muscle cells to become damaged and weakened over time.
VYONDYS 53 uses exon-skipping technology to allow the body to make a shorter form of the dystrophin protein in some patients by skipping over a specific exon on the dystrophin gene.
Meet Nicholas, age 17
Amenable to exon 53 skipping
“The Duchenne patient’s genetic test identified that he had a mutation that could be treated by VYONDYS 53. His doctor said he was amenable to treatment with the exon-skipping therapy.”
What is exon skipping?
Duchenne is caused by a genetic mutation in the dystrophin gene. Most commonly, one or more exons (parts of the gene) are missing, causing errors in the instructions for making dystrophin. This results in the body not being able to produce enough—or any—working dystrophin protein. The goal of exon skipping is to allow the body to make a shorter form of the dystrophin protein. This video will show you how.
How exon skipping works.
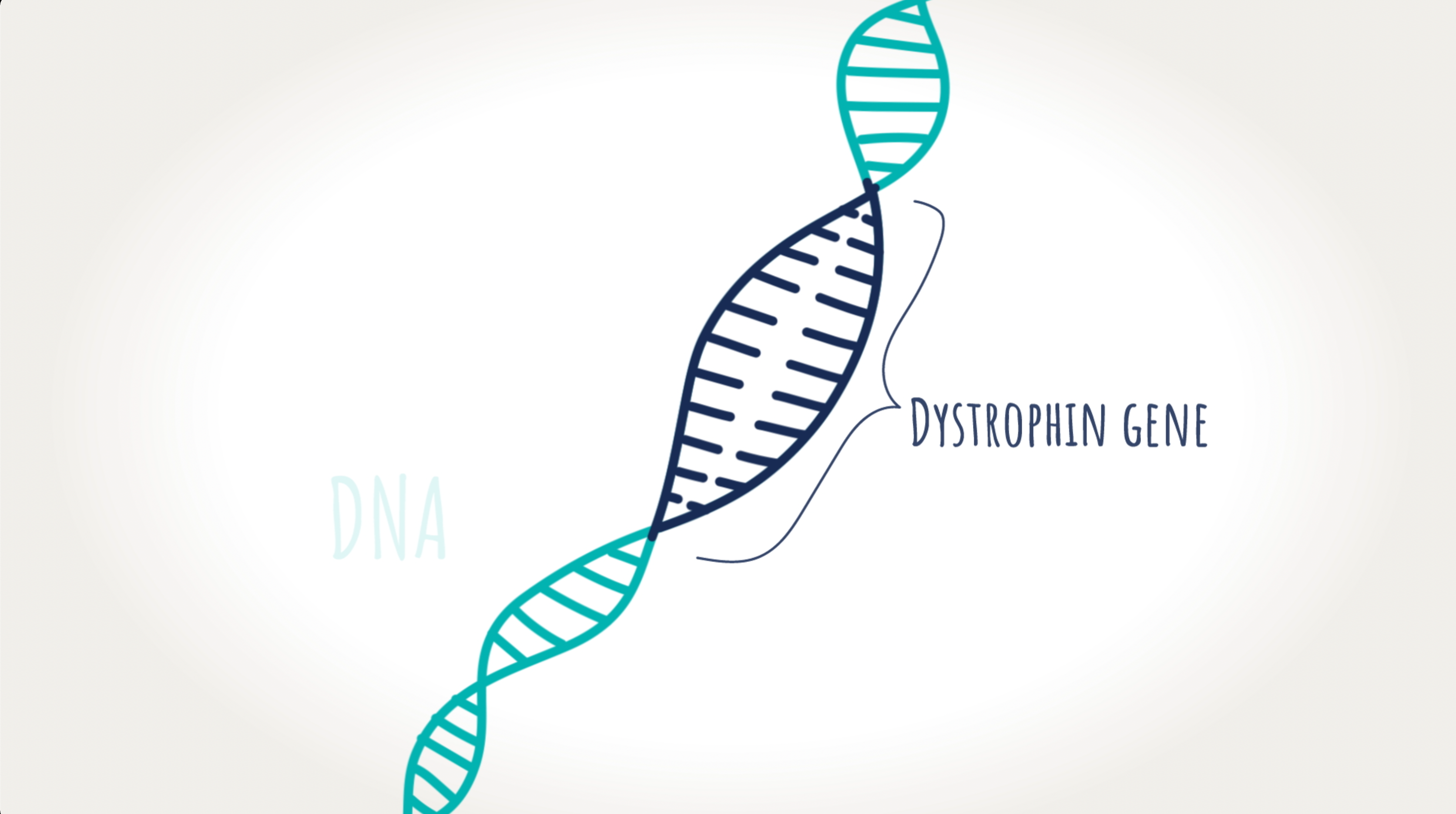
Understanding the dystrophin gene.
The dystrophin gene is made up of exons (portions of a gene) that are linked together to provide instructions for making dystrophin—a protein our muscles need to work properly. Without dystrophin, muscle cells become damaged and weaken over time.
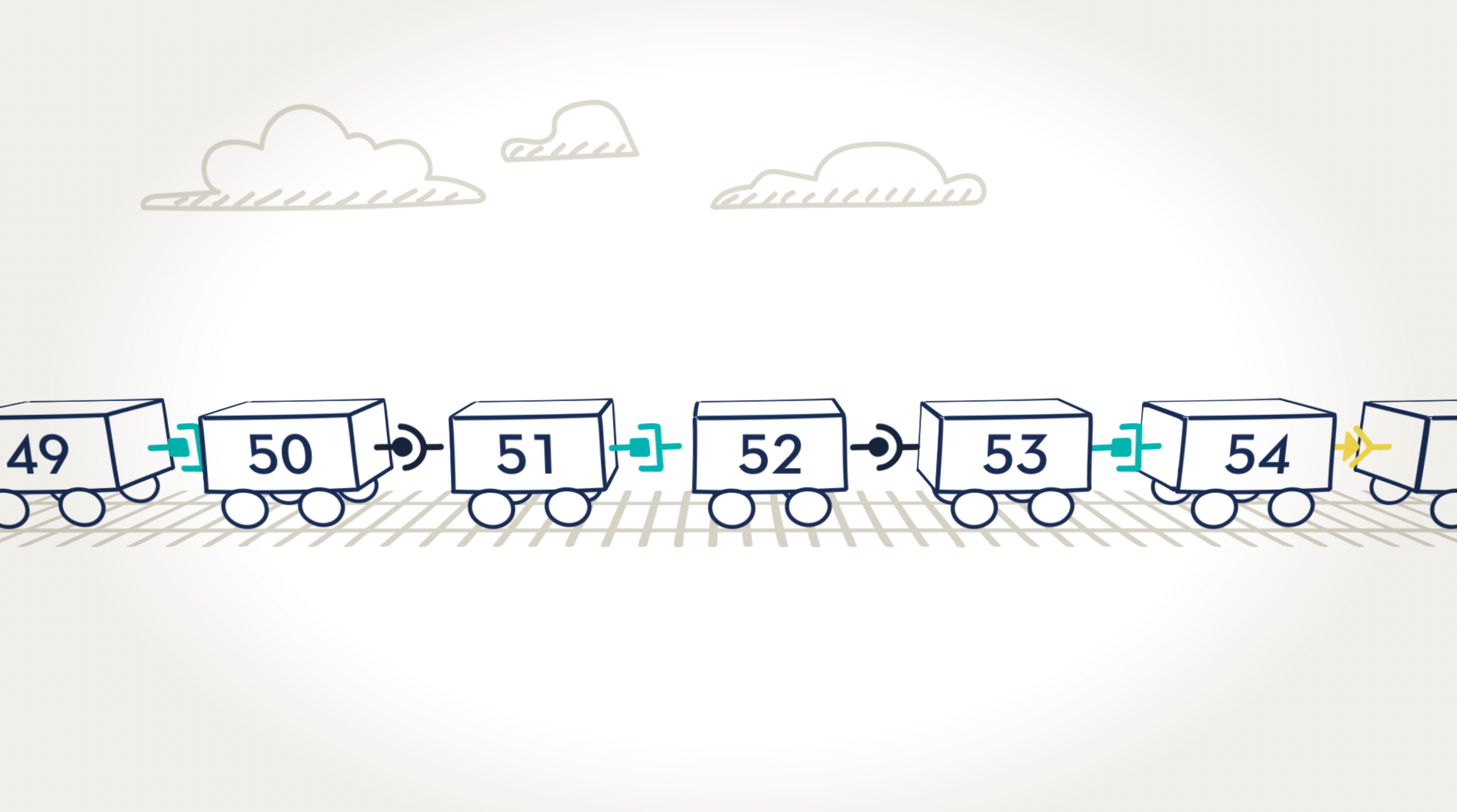
How exons connect.
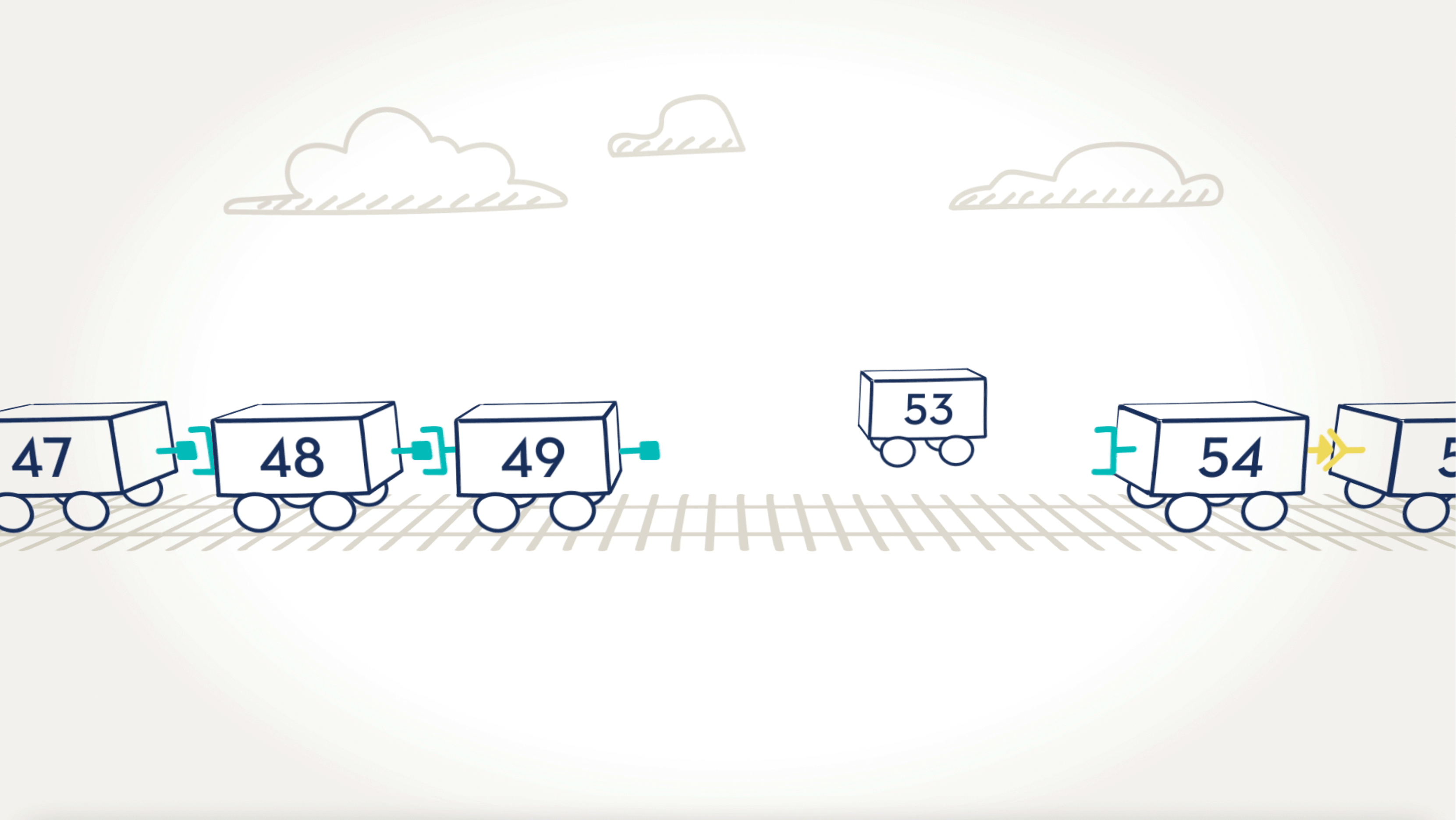
With 79 exons, the dystrophin gene is the largest in the body. Think of the exons on the dystrophin gene like toy train cars, each with a special connection that allows one car to connect to another. In order for all the cars to move together as a train, the connections between cars must match—for example, circle-to-circle and square-to-square.
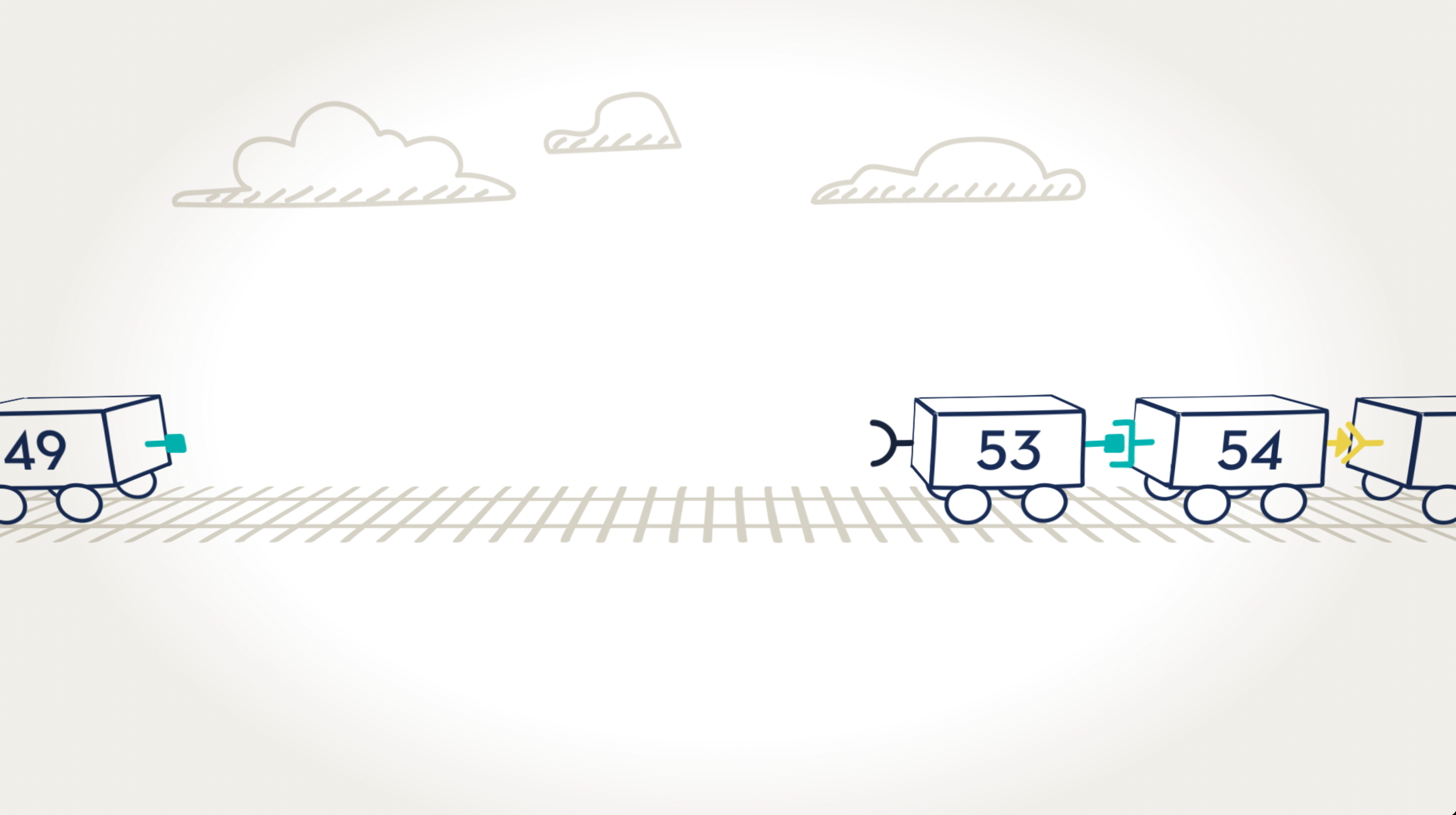
When an exon is missing.
Here we can see that cars, or exons, 50, 51, and 52, are missing. The result is that cars 49 and 53 are not able to connect because their connectors are different shapes and don’t fit together. In the dystrophin gene, this missing exon would prevent the body from being able to read the instructions for making the dystrophin protein.
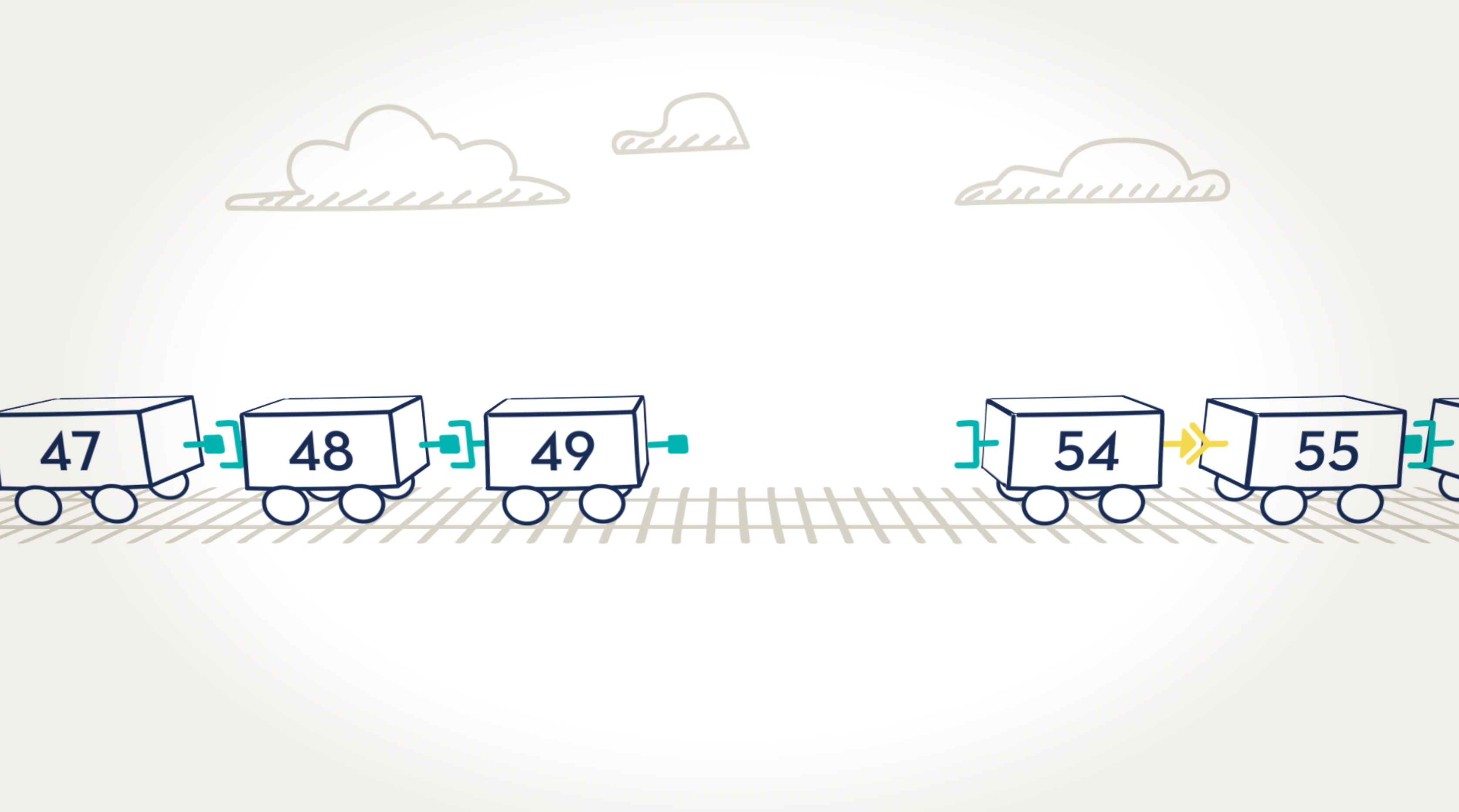
Skipping over exons.
By moving certain exons—or cars—aside, we can “skip over” them to find a car with the right connector. The new train would be shorter, but the cars are connected. Just as we skipped over a train car, VYONDYS 53 is designed to skip over an exon.
The result: A shorter form of dystrophin.
VYONDYS 53 works using exon-skipping technology. In some boys, weekly infusions with VYONDYS 53 have been shown to help the body make a shorter form of the dystrophin protein. Watch the video.

Clinical studies of VYONDYS 53 tested whether exon skipping happened on the dystrophin gene of boys treated with the drug. In those studies, exon skipping occurred in all 25 evaluated study participants. Boys who received VYONDYS 53 had variable responses in the amount of dystrophin production.
Related FAQs
Do not receive VYONDYS 53 if you are allergic to golodirsen or any of the ingredients in VYONDYS 53. Serious allergic reactions to golodirsen have included anaphylaxis, which may include difficulty breathing and tightness in the chest.
Yes. VYONDYS 53 has been studied in clinical trials. See the results from clinical trials.
Duchenne patients who receive VYONDYS 53 must have a genetic test that shows a mutation in the dystrophin gene that can be treated by skipping exon 53. Your child's doctor is best equipped to determine if your child’s mutation is amenable to treatment with VYONDYS 53. We’ve developed a Doctor Discussion Guide to help you start that important conversation.
